This article, & the book Sacred Cacti, is best viewed at http://sacredcacti.com/blog/lophophora-williamsii-analysis/ My apologies for any 404 pages that may exist as the transfer is completed.
An interesting objection to peyote cultivation has been raised based on the assertion that peyote in cultivation may not express all of the alkaloids reported from wild plants. Something which was missed in this claim is that relatively few alkaloids have been reported from wild peyote and the majority, including all of the known trace alkaloids, were found using elaborate gc-ms trapping experiments and other approaches intended to capture short-lived intermediates and trace alkaloids. All of those studies used peyote plants which had been grown from seed and cultivated in greenhouses, primarily in northern Europe. rather than wild harvested plants.
Reported analysis of Lophophora williamsii
Mescaline content of Peyote
As is true for the alkaloid level of any plant, the mescaline content of peyote exists as a range that is influenced by at least several factors. The following simply summarizes the literature. Only some type of actual analysis or bioassay can say something accurate concerning a plant in front of a viewer. Literature should be viewed with caution and regarded to be only guidelines suggesting potential values.
“Arthur Heffter, a German pharmacologist of the nineteenth century estimated that there are about 4.6 to 5.8 grams of mescaline in every kilogram of dried peyote.” (Anderson 1980)
Heffter reported a maximum recovery in his work of 6.3% mescaline, 5.3% anhalonidine, 3% anhalonine, 0.5% lophophorine, 5.3% anhalamine.
Späth later reported having much lower yields working with old material.
Mescaline has been reported from L. williamsii with a min and max value of 0.10% and 6.3%. A range of 0.9-6.0% by dry wt is what is generally given. [Anonymous 1959, Heffter 1896a, Lundström 1971b, Martin & Alexander 1968, McLaughlin & Paul 1967 & Siniscalco 1983);
Anderson 1980 cited Kelsey 1959 (0.9%), Bergman 1971 (1.5%), Fischer 1958 (3%), Heffter 1896a (4.6-5.6 %[-6.3%])
Crosby & McLaughlin 1973 commented that mescaline content in dried peyote can reach 6% but rarely exceeds 1% in dried whole plant.
6% appears in Anderson, Kapadia & Fayez, Lundström 1971b, Martin & Alexander 1968, and Reti 1950. These are all second-hand accounts of that 6% value; referring to its publication by Heffter.
0.1% dry wt is the lowest value in the literature; reported in Siniscalco Gigliano 1983.
Ott 1993 estimated 2.4-2.7% mescaline by dry weight (~400 mg. per 16 grams of dried cactus) citing Bruhn & Holmstedt 1974 and Lundström 1971b.
Friends with extraction experience found fresh plants to average 0.2% mescaline from fresh plants and 1-2% from dried material. This refers to peyote originating from South Texas during the mid-1970s. This work was always done under fairly primitive and inefficient conditions. 2% is usually cited as an estimate in counterculture drug manufacturing literature. (50 grams of dried peyote per gram of mescaline recovered.).
Recently, a meme of “1% max” has been circulating; perhaps reflecting the current decrease in the average age and size of harvested plants due to careless overharvesting and harvest practices?
75-125 mg of HCl was recovered from 70-140 gm plants greenhouse grown in northern Europe. Lundström & Agurell 1971b (This approaches 0.1% by fresh weight; ; 0.1 to 0.2% by fresh weight is a commonly reported range.) [Also in Habermann 1978a & 1978b (from Štarha nd)]
Mescaline has been reported to comprise around 30% of the total alkaloid content of L. williamsii: Lundström 1971b.
Container grown plants in Italy were reported to contain 0.255% by fresh weight (2.55 mg/gm fresh was an average value derived from two specimens; estimated using HPLC). They also reported an average of 1.75% by dry weight. (Ed.: Note the obvious discrepancy)
Gennaro et al. 1996;
As L. williamsii var. typica Croizat:
0.709% (± 0.032) dry wt. Habermann 1978a (from Štarha 1997)
Variations across range:
Starr Co.: 2.77%;
Jim Hogg Co: 3.2%;
Val Verde Co: 3.5%;
Presidio Co: 3.52%.
(Averaged % by dry weight: – Used batched samples.
Hulsey et al. 2011.
Regrowth:
3.80% mature crowns,
2.01% small regrowth crowns (4 year after the prior harvest).
(Jim Hogg Co. Averaged % was by dry weight: – Used batched samples.)
Kalam et al. 2012 & 2013.
Batched samples were used to deliberately create an average value and lessen the possible contribution from potential high or low outliers. Comparison of Hulsey with Klein’s paper shows the wisdom in choosing that approach even if it does deprive us of an understanding of the max/min values. The ideal approach is a screening using batched plants followed by a more detailed look at a set of the individuals.
One peer reviewer suggested that batching in Kalam invalidated their results, which if true would invalidate the results of almost all published analytical work appearing in the history of phytochemistry. Almost all workers analyze multiple individuals to minimize the influence of potential outliers, the only actual difference between the acceptable approach of those workers (including in the same journal) and what was complained about with Hulsey or Kalam is the earlier workers did not REFER to their batched samples as being a batched sample.
Distribution in the peyote plant
Janot claimed to have established that mescaline was largely produced in the peripheral green parenchyma of the crown. As this was during the 1930s the identification would have been established using microchemical methods.
Todd 1969 found mescaline in the tops to be substantially greater than in the roots (using co-TLC). (See Note B)
Anonymous 1959, citing Rouhier 1927 “Le Peyotl”, gives the following percentages of alkaloid content in different parts of the cactus (% by dry weight unless otherwise stated):
Upper slices dried 3.70%
Lower slices dried 3.43%
[The above refers to the practice sometimes employed of horizontally sectioning the top of the cactus into two parts prior to drying.]
Peyote head dried 3.14%
Fresh peyote head 0.41%
Roots dried 0.73%
Fresh roots 0.244%
A closer look using 13 individual plants divided into three parts (crown, stem & root) that were then each analyzed separately:
1.82-5.50% in crown tissue,
0.125-0.376% in subterranean stem tissue,
0.0147-0.0520% in root tissue.
(Starr Co.; Analyzed individually. All % by dry wt.)
Klein et al. 2013 & 2015.
Notice that there is an order of magnitude decrease from crown to stem and again from stem to root?
Growth conditions
Siniscalco Gigliano 1983 reported his isolation of mescaline as:
0.10% from well irrigated plants,
0.93% from his grafted plants, and
up to 2.74% dry weight after 6 months of dry conditions.
All from peyote plants being cultivated in Italy.
Dried plant is said to have 3% Roland Fischer but Fischer claimed that only if chewed well or ground finely can this be extracted. He presented a study as indicating that less than one percent is obtained by chewing and swallowing. While finely grinding or chewing well is important for obtaining the best possible absorption (especially if using dry material) it must be pointed out that Fischer’s reasoning had some problems.
Fischer was able to get 3% mescaline from dried peyote by grinding it to a powder before beginning his extraction procedure. He found that if this dry grinding was omitted and the buttons rehydrated by soaking in water for two hours and then ground before extraction he could only recover 1%.
He went on to conclude “The only safe conclusion would be that the chewing of peyote and the swallowing as a bolus are certainly less thorough extraction procedures than our “wet grinding” procedure which recovers only about 1% of mescaline.”There several major flaws in Fischer’s reasoning and procedure, in so far as applying it comparatively to humans.
The more trivial of the two concerns Fischer basifying the buttons after soaking in water and then grinding, filtering, washing and adjusting the pH to 3.4 to 4.
In the stomach the chewed buttons are repeatedly macerated and massaged by peristaltic contractions in a dilute but fairly strong solution of hydrochloric acid (normally pH 1.5 to 2.5) which converts the rather poorly soluble mescaline base into the exceedingly water soluble mescaline hydrochloride. This means that the acidity used in human in vivo extraction is several orders of magnitude greater than that used by Fischer. (The effects of the digestive enzymes in the stomach do not contribute much as they consist primarily of pepsin which is specific for proteins.)
A more significant point was Fischer’s choice of base. When recovering 3%, he had used sodium hydroxide to bring it to pH 8.6, which is nearing the lower limit for good mescaline extraction as the free base. (97% at pH 8.6 according to Woods et al. 1951; 100% extraction is said to occur at pH 9 or above.). When he recovered 1%, for some reason he had decided to use sodium carbonate instead. This base is a good choice for many alkaloids. (It would have been acceptable if, for example, he was isolating DMT.) His bringing the pH to 8.8 might have enhanced his yield a trivial amount but mescaline has a tendency to form an insoluble carbonate, whether the carbonate source is in air or solution. This may have decreased his yield. [This may also have caused Reti some loss with Trichocereus terscheckii as well. This is just a hunch as rigorous evaluation has not been conducted. I should add that the presence of CO2 is also said to be critical for crystallization of mescaline to occur; according to LaBarre 1989.]
Although in agreement with the idea that chewing well or fine grinding is important to the best absorption, any direct comparisons of his findings to human rates of internal utilization need questioning.
While direct measurements of internal absorbence may not be possible, it would be feasible to administer known dosages of mescaline and subjectively compare them with known amounts of mescaline in cactus material. If a series of such bioassays were performed using experienced users a rough estimation could be determined which would be at least as accurate as Fisher’s extrapolation. It may also be possible to determine the percent of absorbence by monitoring the initial rise in blood levels during the early stages. This also would require the use of pure mescaline to establish a baseline. It also would require repeated evaluations using both different and the same individuals to be certain that biochemical individuality did not affect the results.
There are two additional accounts in the literature that are important to be aware of:
Sasaki et al 2009 and Aragane et al. 2011 published details from an interesting study of Lophophora demonstrating that genetic and chemical differences exist between L. williamsii and L. diffusa.
They additionally included three specimens of L. fricii but apparently renamed it based on what they found in publications by Edward Anderson, by Yoshio Ito & by H. Hirao. Aragane presented it to be a nonmescaline variant of L. williamsii. Earlier, Sasaki had said they had reidentified it as L. williamsii var. decipiens.
It is clear without any doubt that those three specimens were Lophophora fricii.
Aragane noted them to differ from their Lophophora williamsii:
1) by the word grey appearing only in the descriptions of their body color and not in those of any of their L. williamsii,
2) bearing large protuberences on the epidermis rather than small ones,
3) 2 of the 3 were noted to have a darker pink flower,
4) The primer sets were different from all of their L. williamsii (and closer to what was noted for diffusa),
5) They were purchased identified as Ginkangyoku (which is the Japanese trade name for L. fricii).
I’ll quote from those two papers as their contained comments provide more than adequate support for my line of reasoning:
“We identified the materials according to Anderson’s morphological classification.” Sasaki et al 2009.
The pertinent point being that Anderson recognized both Habermann’s Lophophora fricii (and wild plants he had encountered of Lophophora koehresii) to be L. williamsii. His view that only two species exist (L. diffusa and L. williamsii) is the basis for Aragane & Sasaki’s name assignment.
“Although the presence or absence of mescaline can easily be checked by chromatography, it is difficult to identify the species because not all L. williamsii contain mescaline. Chemotaxonomic identification of L. williamsii seems insufficient. DNA sequences of chloroplast trnL intron region in Lophophora plants were revealed to be beneficial for identification and showed a good correlation with mescaline content.” Sasaki et al. 2009.
“These samples were identified as L. williamsii in this study but were identified as L. williamsii var. decipiens in the literature.” [citing two illustrated cactus books by Y. Ito and by H. Hirao) Sasaki et al. 2009
“Interestingly, although Lo-14, Lo-15, and Lo-16 were identified as L. williamsii in this study, these three samples were also identified as L. williamsii var. decipiens in previous literature. 16,18
Sequence alignments in the trnL intron region of those three samples were different from those of Lo-2 to Lo-11. Moreover, another study of ours revealed that Lo-14, Lo-15, and Lo-16 contained no mescaline (Table 1). Using this method, we can distinguish mescaline-containing Lophophora plants from mescaline-free ones if the reaction is stopped at 65 min.”
Sasaki et al. 2009 (Lo-14, Lo-15, and Lo-16 were their Lophophora fricii specimens. Lo-2 through Lo-11 were all L. williamsii.)
“morphology of Lo-14 to 16 was similar to that of L. diffusa (Lo-17 to 20).” Aragane et al. 2011
“It was reported that L. williamsii contained mescaline, but that L. diffusa did not [15, 16]; however, it was unknown whether that L. williamsii was within the wide classification that included L. fricii. In this study, we clarified for the first time that there are two groups of L. williamsii, one with mescaline (group 1) and the other without it (group 2), and that L. diffusa contained no mescaline.” Aragane et al. 2011
It is fascinating that they did not grasp that they had just produced might could be considered to be adequate proof that L. fricii merited recognition as a species separate from L. williamsii rather than being considered to be a nonmescaline form of L. williamsii.
Aragane et al. 2011 reported mescaline concentrations in their Japanese horticultural specimens to range from 1.27-4.83%. (The concentrations reported for those 13 averages to 3.2%.) This is a range AND an average value that is quite comparable to what has been reported from wild plants.
Their presented sources produced some questions. In Sasaki et al. 2009 their plants were said to have been obtained from the “Medicinal Plant Garden, Tokyo Metropolitan Institute of Public Health“. In Aragane et al. 2011, most were listed as having been acquired through the “Internet” with all of the remainder coming from “Market (Mie Pref.)“.
Whether Aragane’s comments on dates and sources referred to the origin for the plants that Sasaki listed as being from the “Medicinal Plant Garden, Tokyo Metropolitan Institute of Public Health” or if Aragane’s comments were intended as a correction to Sasaki is not made clear.
These are the results from Aragane et al. 2011 concerning their specimens that were actually Lophophora williamsii:
| # | Mescaline | Name | Date | Source |
| Lo-1 | 3.72% | Ubatama | 4-2005 | Internet |
| Lo-2 | 4.83% | Ubatama | 4-2005 | Internet |
| Lo-3 | 2.22% | Ubatama | 1-2005 | Market (Mie Pref.) |
| Lo-4 | 4.27% | Ubatama | 1-2005 | Market (Mie Pref.) |
| Lo-5 | 3.85% | Ubatama | 1-2005 | Market (Mie Pref.) |
| Lo-6 | 2.62% | Ubatama | 4-2005 | Internet |
| Lo-7 | 3.82% | Ubatama | 4-2005 | Internet |
| Lo-8 | 2.46% | Ubatama | 4-2005 | Internet |
| Lo-9 | 2.94% | Ubatama | 4-2005 | Internet |
| Lo-10 | 3.07% | Ubatama | 4-2005 | Internet |
| Lo-11 | 3.54% | Ougataubatama | 4-2005 | Internet |
| Lo-12 | 2.5% | Kofukiubatama | 3-2005 | Internet |
| Lo-13 | 1.27% | Ougataubatama | 4-2005 | Internet |
Alkaloid content of Peyote:
Of the total alkaloid content:
30% is present as mescaline; 17% as pellotine.
Schultes & Hofmann 1980: 221.
Total alkaloid reported:
8.41% in dried “buttons”;
0.47% in fresh whole plants;
0.2% in fresh roots
0.93% in fresh tops.
Bruhn & Holmstedt 1974.
See more farther below.
Lewin was the first to isolate an alkaloid from peyote but it turned out to be both inactive entheogenically and a mixture of several alkaloids.
Heffter isolated 3 alkaloids from Lophophora williamsii and published his results and pharmacology in 1898. He named the active compound mescaline; determining it to be the active alkaloid by personal bioassays. [Heffter 1898a] Heffter named the other two alkaloids Anhalonidine and Lophophorine.
In 1976, 50 alkaloids had been observed;
(29 as substituted phenethylamines and 23 as tetrahydroisoquinolines):
Shulgin 1976 cited Kapadia & Fayez 1973
A total of 35 isoquinolines had been reported prior to 1986, according to Menachery et al. 1986.
The number of compounds now mentioned in the chemical literature as actually being detected in the plant is 72. Of which some are questionable inclusions, some are clearly errors and a number alkaloids still need a second-party confirmation by someone. At the moment the presence of 63 alkaloids has been established.
No doubt new trace alkaloids in peyote will continue to be found in the future so long as people devise more sophisticated techniques and/or continue to look for them.
It should be pointed out that any and all recent finds of alkaloids have been in trace quantities. Most have been identified using elaborate ‘trapping’ techniques for identifying short-lived biosynthetic precursors. Which also means it is a bit of a stretch to consider those components in the alkaloid fraction since normal extraction processes will not be able to recover them.
Any alkaloids discovered in the future will similarly be of purely biochemical interest rather than pharmacological contributors to the action of peyote.
In an incredible move suggested more than a small level of ignorance (and, at best, a serious lack of factual information), in 1997 Congress made law a provision declaring every alkaloid contained in peyote to be a Schedule One controlled substance.
Since several of these are normal components of human body fluids (including blood, CSF and urine) and many are present in a wide variety of plants, what this actually means remains to be seen.
It is more than a bit disconcerting that there are now AT LEAST a handful of normally present endogenous substances that are presently considered Schedule 1 (potentially as many as 9 different compounds); placing every human on the planet in measurable and perennial violation of US federal law.
According to Anderson 1980, Todd found little variation in the alkaloid concentration between roots and tops of plants except for hordenine which he found to be present only in the roots. This is misleading as stated.
Todd 1969 analyzed two populations of Lophophora williamsii (and also L. diffusa from Querétaro) collected during June, [a time considered to be poor for mescaline and good for isoquinoline effects.] His collections were made by Anderson near Monclova, Coahuila and El Huizache, San Luis Potosí.
Todd found lophophorine to be present at higher concentrations than mescaline in the plants collected from both locations. [It has been noted by other workers that N-Methylated compounds, such as Lophophorine, are higher during summer than winter. See below.]
Anhalamine and anhalonidine were present at nearly the same concentration as mescaline in plants collected in Coahuila and at the same concentration as mescaline in plants collected from San Luis Potosí.
Anhalonine and anhalinine were present at about half the concentration of mescaline in both populations.
While pellotine in the tops was present at lower concentrations than mescaline in the Coahuilan population, it was present at roughly equal concentrations to mescaline in the San Luis Potosí population.
Mescaline concentrations were found to be substantially higher in the population collected from Coahuila.
The difference in mescaline concentration between the roots and tops was found to be far greater in plants from San Luis Potosí than Coahuila. The mescaline concentration in the roots of Coahuilan plants was equal to the concentration of mescaline in the tops of the San Luis Potosí originating plants. Only traces of mescaline were observed in the roots of the San Luis Potosí originating plants (the Texas ‘Peyote Gardens’ population is believed to be similar).
Pellotine was found to be equally distributed between roots and tops in both populations but was present in higher amounts in the San Luis Potosí population.
Anhalamine, anhalonidine, and anhalonine were found to be equally distributed between roots and tops and were present in similar concentration in both populations.
Anhalinine and lophophorine were found to be equally distributed between tops and roots in the population at San Luis Potosí and less concentrated in the roots of those from Coahuila. Concentration in the tops of both populations were the same.
I suspect that it was the collection during June that caused the marked differences between his results and those of other investigators. A similar examination should be made using collections taken at two month intervals during December through mid-May, the usual time of indigenous people’s collection for use. The isoquinoline content proportional to mescaline, as reported by Todd, is far higher than is normally mentioned in the literature. [All of Todd’s concentrations were estimated by co-tlc with known amounts.]
The Coahuilan population is considered to be a stronger variety or even a separate species by some. Chemically there may be justification for this [Note 21] and it should be targeted for propagation. Plants originating from the Texas “peyote gardens” are believed to be similar to the San Luis Potosí population.
Todd’s descriptions do not allow comparison with the published descriptive differences between var. williamsii and var. echinata.
Lundström 1971b reported that the N-methylated alkaloids (such as Lophophorine) were highest during summer in greenhouse maintained plants. N-Demethylated compounds were found to be higher in fall and winter than N-methylated derivatives.
This corresponds well to Peyote using peoples traditionally gathering plants from November through April or mid-May (actual period of harvest varying from group to group but largly falling within this time frame with thre being at least one group of Huichols harvesting in October) and also with subjective observations that December through early March are the times for the best psychological effects and the least somatic distress. I believe that January and February are the most ideal months of the year.
Siniscalco 1983 reported that keeping cultivated peyote plants under arid conditions for 6 months substantially increased their mescaline content. Their corresponding reported values differed as 0.1% compared to 2.74% by dry wt. That is a 27.4X difference which is highly significant as taking a plant from fresh to total dryness only increases the concentration ~10X.
In whole fresh plants of L. williamsii, a total alkaloid content of 0.47% was found. (Of this 60% was present as phenolic alkaloids and 40% as nonphenolic alkaloids.)
The fresh roots had a total alkaloid content of 0.20% (67% phenolic/ 33% nonphenolic). The fresh tops had a total alkaloid content of 0.93% (58% phenolic / 42% nonphenolic)
Plants were harvested in ?? (they mentioned that L. diffusa was harvested in June).
[They added that Lundström 1971b found 0.4% total alkaloids in whole plants of which 57.5% was phenolic and 42.5% was nonphenolic alkaloids.]
Analysis of old materials
Dried peyote buttons, freshly prepared, had a total alkaloid content of 8.41% (64% phenolic versus 36% nonphenolic).
87 year old peyote buttons (sent to Watson by Rusby in 1887) had an alkaloid content of 8.86% (65% phenolic and 35% nonphenolic).
The mescaline content of the 87 year old buttons was much less than the new ones but they did not have enough variables to account for the difference. Only minor differences were observed with regards to most of the other alkaloids. Anhalinine was also markedly lower in the old material. Hordenine and 3-Hydroxy-4,5-dimethoxyphenethylamine were almost completely lacking from the old material. The latter of these had been noted earlier by both Späth 1922 and Agurell & Lundström 1968 as being rather unstable.
Bruhn & Holmstedt 1974
Percentages of alkaloids reported in peyote:
Ott 1993 gave a nice summary; citing Bruhn & Holmstedt 1974 and Lundström 1971b:
8% Total alkaloids in dried peyote buttons, of which:
30% is mescaline (= 2.4-2.7%) (~400 mg. per 16 grams of dried cactus)
17% is pellotine (peyotline) (= 1.4-1.5%)
14% anhalonidine (= 1.2-1.3%)
8% anhalamine (= 0.6-0.7%)
8% hordenine (= 0.6-0.7%)
5% lophophorine (= 0.4%)
Alkaloid percentages according to Kapadia & Fayez 1973.
References cited are theirs. (All percentages of total alkaloid content are from Lundström 1971)
Mescaline 6% (30% of total alkaloid content.)
Anonymous 1959
Pellotine (peyotline) 0.74% (17% of total alkaloid content.)
Heffter 1894b [This may have been from L. diffusa.]
Anhalonidine 5% (14% of total alkaloid content.)
Heffter 1896a
Anhalamine 0.1% (8% of total alkaloid content.)
Heffter 1901
(Späth & Becke 1935b also reported 0.1%.)
Lophophorine 0.5% (5% of total alkaloid content.)
Heffter 1896a
Anhalonine 3% (3% of total alkaloid content.)
Heffter 1896a
Anhalinine 0.01% (0.5% of total alkaloid content.)
Späth & Becke 1935a & 1935b
Anhalidine 0.001% (2% of total alkaloid content.)
Späth & Becke 1935a & 1935b
Hordenine 0.004% (8% of total alkaloid content.)
N-Methyl-4-hydroxy-3-methoxyphenethylamine (<0.5% of total
alkaloid content.)
N,N-Dimethyl-4-hydroxy-3-methoxyphenethylamine (0.5-2% of total alkaloid content.)
3-Demethylmescaline (1-5% of total alkaloid content was found in fresh material by Lundström & Agurell 1971)
N,N-Dimethyl-3-demethylmescaline (0.5% of total alkaloid content.)
N-Methylmescaline 0.002% (3% of total alkaloid content.)
O-Methylanhalonidine (<0.5% of total alkaloid content.)
Isopellotine (0.5% of total alkaloid content.)
Peyophorine (0.5% of total alkaloid content.)
Isoanhalidine (trace)
Isoanhalonidine (trace)
Isoanhalamine (trace)
Tyramine (trace)
N-Methyltyramine (trace)
Epinine (trace)
3,4-Dimethoxyphenethylamine (trace)
3,4-Dihydroxy-5-methoxy-phenethylamine (trace)
N-methyl-3-demethylmescaline (trace)
[All others found by other workers were also trace components.]
For more information on isolations and dates see elsewhere here.
Lundström 1971b found a total alkaloid content of 0.4% w/w to be present in the fresh buttons and noted that 0.41% had been determined by Rouhier (as cited by Anonymous 1959).
First pharmacological study of peyote was published in Lewin 1888a & 1894a.
An Abbreviated Chronology of the Identification of the Peyote alkaloids
The first report of alkaloids in peyote was the laboratory report of F.A. Thompson at Parke-Davis but Lewin was the first to publish. (Bruhn & Holmstedt 1974)
1888
Anhalonine (crystalline but not a pure compound)
Lewin (1888) Naunyn-Schmiedebergs Archiv fur Experimentelle Pathologie und Pharmakologie, 24: 401-411
1894
Pellotine This probably was from L. diffusa rather than L. williamsii. [The source of Heffter’s material is not known as this apparently came from German collectors with no identification of locality. Considerable trade of peyote collected from the locality of L. diffusa existed in early times and it was not differentiated from L. williamsii so it is probable that pellotine was not actually isolated from L. williamsii by Heffter. He referred to the material in this analysis as Anhalonium williamsii rather than A. lewinii, the latter being his source of mescaline below. See Bruhn & Holmstedt 1974 or the A. lewinii discussion herein.]
Heffter (1894)b Berichte der Deutschen Chemischen Gesellschaft, 27: 2975-2979.
1896
Anhalonidine
Lophophorine
Mescaline
Heffter (1896)a Berichte der Deutschen Chemischen Gesellschaft, 29: 216-227.
1899
Anhalamine
Kauder (1899) Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft, 237: 190-198.
1935
Anhalinine
Späth & Beck (1935) Berichte der Deutschen Chemischen Gesellschaft, 68 (3): 501-505.
Anhalidine
Späth & Beck (1935) Berichte der Deutschen Chemischen Gesellschaft, 68 (5): 944-945.
1937
N-Methylmescaline
Späth & Bruck (1937) Berichte der Deutschen Chemischen Gesellschaft, 70 (12): 2446-2450.
1938
N-Acetylmescaline
Späth & Bruck (1938) Berichte der Deutschen Chemischen Gesellschaft, 71 (6): 1275-1276.
1939
O-Methylanhalonidine
Späth and Bruck (1939) Berichte der Deutschen Chemischen Gesellschaft, 72 (2): 334-338.
1965
Hordenine
McLaughlin & Paul (1965) Journal of Pharmaceutical Sciences, 54 (4): 661.<
(Confirmed in McLaughlin & Paul 1966 Lloydia, 29 (4): 315-327.)
See Todd 1969 Lloydia, 32 (3): 395-398.
1966
Tyramine
N-Methyltyramine
Candicine (Identified by tlc. Presence in peyote is in question, see Kapadia et al. 1968 Journal of Pharmaceutical Sciences, 57 (2): 254-262.)
McLaughlin & Paul (1966) Lloydia, 29 (4): 315-327. (In addition to hordenine)
1967
Peyonine
Kapadia & Shah (1967) Lloydia, 30: 287. (Proceedings.)
See also Kapadia & Highet (1968) Journal of Pharmaceutical Sciences, 57: 191-192
1968
3-Hydroxy-4,5-dimethoxyphenethylamine
Agurell & Lundström 1968 The Chemical Society, London. Chemical Communications, 1638-1639.
(Confirmed by Kapadia et al. (1969)a Journal of Pharmaceutical Sciences, 58 (9): 1157-159.)
N-Acetylanhalamine
N-Acetylanhalonine
N-Acetyl-3-hydroxy-4,5-dimethoxyphenethylamine
N-Formylanhalamine
N-Formylanhalinine
N-Formylanhalonidine
N-Formylanhalonine
N-Formyl-3-hydroxy-4,5-dimethoxyphenethylamine
N-Formylmescaline
N-Formyl-O-methylanhalonidine
Mescaline maleimide
Mescaline malimide
Mescaline succinamide
Mescalotam
Peyoglutam
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
Peyophorine
Kapadia & Fales (1968)b Journal of Pharmaceutical Sciences, 57 (11): 2017-2018, and Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications,24: 1688-1689.
Anhalotine (as iodide)
Choline
Lophotine (as iodide)
Peyotine (as iodide)
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
3,4-Dimethoxyphenethylamine
Lundström & Agurell (1968) Journal of Chromatography, 36 (1): 105-108.
1969
Peyoxylic acid
Peyoruvic acid
Kapadia et al. (1969) Paper presented at the 116th Meeting of the American Pharmaceutical Association, Montreal, Canada. May 18-22, and Kapadia et al. (1970)b Journal of the American Chemical Society, 92 (23): 6943-6951.
1970
Mescaline citrimide
Mescaline isocitrimide lactone
Kapadia & Fales (1970)a Lloydia, 33 (4): 492. (Proceedings.) (Paper presented at the “11th Annual Meeting of the American Society of Pharmacognosy (Vienna, Austria) July 1970)”
Peyoglunal
Kapadia et al. (1970)a Lloydia, 33 (4): 492. (Proceedings.)
1971
Mescaloxylic acid
Mescaloruvic acid
Kapadia et al. (1971) Paper presented at the 118th Meeting of the American Pharmaceutical Association, San Francisco, California, March 27-April 2. “Some newer synthetic cactus alkaloid analogs.” and Kapadia and Hussain (1972) Journal of Pharmaceutical Sciences, 61 (7): 1172-1173.
Dopamine (3,4-Dihydroxyphenethylamine)
Epinine (N-Methyl-3,4-dihydroxyphenethylamine)
4-Hydroxy-3-methoxyphenethylamine
N-Methyl-4-hydroxy-3-methoxyphenethylamine
N,N-Dimethyl-4-hydroxy-3-methoxyphenethylamine
N-Methyl-3,4-dimethoxyphenethylamine
3,4-Dihydroxy-5-methoxyphenethylamine
Lundström (1971) Acta Chemica Scandinavica, 25 (9): 3489-3499.
N,N-Dimethyl-3-hydroxy-4,5-dimethoxyphenethylamine
N-Methyl-3-hydroxy-4,5-dimethoxyphenethylamine
Lundström (1971) Acta Pharmceutica Suecica, 8: 485-496.
1972
6,7-Dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt
1,2-Dimethyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt
1-Methyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinoline
2-Methyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt
Fujita et al. (1972) Yakugaku Zasshi, 92 (4): 482-489
Isoanhalamine
Isoanhalidine
Isoanhalonidine
Isopellotine
Lundström (1972) Acta Chemica Scandinavica, 26 (3): 1295-1297.
1973
O-Methylpeyoxylic acid
O-Methylpeyoruvic acid
Kapadia et al. (1973) Journal of Heterocyclic Chemistry, 10 (1): 135-136.
1977
Pellotine determined to exist in optically active form in the cactus. (This had been an unresolved question for many years due to rapid and ready racemization)
Cymerman Craig et al. (1977) Journal of the American Chemical Society, 99 (24): 7996-8002.
1996
Serotonin was claimed; using ion-interaction HPLC. Its identity was never actually proven and it was not isolated. It presently lacks confirmation.
Gennaro et al. (1996) Analytical Letters, 29 (13): 2399-2409.
2008
3,4-Methylenedioxyphenethylamine (Homopiperonylamine)
3-Methoxy-4,5-methylenedioxyphenethylamine (Lophophine)
N,N-Dimethyl-3,4-methylenedioxyphenethylamine (Lobivine)
These three compounds were reported but this needs to be taken with caution as their actual isolation and characterization was never performed. All identifications relied entirely on the spectral data of the extracted alkaloids and their corresponding derivated forms. The actual presence of these alkaloids still needs to be independently confirmed. A number of comments from this paper also need questioning, especially concerning their peculiar speculative assertions of their contributions to activity and their baseless allusions to MDMA or designer drug activity. (It was incredibly entitled “Ecstacy analogues found in cacti.” as if the activity of MDMA analogs did not require alpha substitution.) In a personal conversation, shortly after the appearance of this paper, Shulgin described the inclusion of his name as an author to be an “embarassment“.
Bruhn et al. (008) Journal of Psychoactive Drugs, 40 (2): 219-222.
Shulgin had however voiced his anticipation, in PIHKAL, that someday someone WOULD find 3-Methoxy-4,5-methylenedioxy-phenethylamine in a cactus and that it was a surprise that it had not been reported already.
Mrs. Anna B. Nickels, a long-time collector of cacti, is generally given credit for bringing peyote to the attention of Parke-Davis. [Safford 1908 is the first source I can find which claims this.]
Slotkin 1955 dismisses this on three counts:
1) Parke, Davis and Co. was unable to find any records concerning Mrs. Nickels,
2) Peyote from Parke, Davis and Co. was used by Lewin, and was said by both sources to have originated in Mexico; Mrs. Nickels lived in Laredo.
3) Mrs Nickels referred to peyote as mescal buttons.
Slotkin presented some circumstantial evidence that J.R. Briggs may have been the one who brought peyote to the attention of pharmaceutical science:
1) Briggs’ brother lived in Mexico and supplied him with peyote.
2) Park, Davis’ files on peyote begin with a clipping of a Briggs article.
3) Both Lewin and Briggs used the unusual name of muscale buttons.
Mrs. Nickels did bring the fact of this plant having medicinal use among native people to the attention of John M. Coulter (around 1892-3). She referred to them as “mescal buttons”.
It might be added that Mrs. Nickels had a large cactus exhibit in Chicago’s 1893 Colombian Exposition and was noted by Liberty Hyde Bailey as having published the first catalog of cacti published in the US (as the price list issued for her cactus retail business ~1876)
A couple of points arise concerning the claims of Slotkin; neither of which am I able to resolve:
Omer C. Stewart was furnished (By G.A. Bender) with a copy of a letter that Mrs. Nickels had sent to Parke-Davis and Company in Detroit dated 11 July 1888.
In this letter, she referred to Anhalonium Williamsii as Piotes.
Bender 1969 presents a somewhat different spin on the same account and presents Parke-Davis as becoming aware of mescal buttons due to reading J.R. Briggs’ published account of his ingestion. In Bender’s account, Briggs was contacted by Parke-Davis and requested to procure some mescal buttons on their behalf, which he eventually accomplished. Interestingly, Parke-Davis apparently lacked any understanding of the nature of their source plant so they sought outside help at identification. One of the dried buttons they had mailed to Lewin in Germany is what ended up in Hennings’ hands and became Anhalonium lewinii.
Effects of peyote summarized
See more details under Mescaline pharmacology (in the book PDF Part C The Cactus Alkaloids) or briefly in the following section.
Perhaps the best summation of peyote’s overall effects to-date was made in 1940 by Richard Evans Schultes:
“Because of the physiological activity of these constituents of the cactus, peyote is capable of inducing an intoxication which is characterized by a feeling of ease and well-being, by control of the limbs and senses, by absence of violence, and occasionally by visual and auditory hallucinations and abnormal synaesthesiae. There are seldom uncomfortable after-effects among users. As a result of this remarkable type of intoxication, peyote has come to be regarded by many Indians as the vegetal incarnation of a deity.” (page 177)
“The sustaining and stimulating properties of Lophophora Williamsii which enable the user to do an excessive amount of work without feeling fatigue are hardly separable from those properties which may be called curative.” (page 178)
Pharmacological overview of the non-mescaline alkaloid content of peyote
No hallucinogenic activity has yet been demonstrated for any peyote alkaloid other than mescaline. [There is one mention of hallucinations experienced with a very large dosage of pellotine and at least one claim of a hallucinogenic experience resulting from the ingestion of L. diffusa but they stand in contrast to all other observations.]
Pharmacology of mescaline and more details concerning the rest of the alkaloids can be found in the book PDF Part C The Cactus Alkaloids. Only a relative few of the peyote alkaloids are mentioned in this section.
Those listed have some nature of activity or lack of activity reported in the literature. Other alkaloids present in peyote, such as anhalinine are unlikely to contribute substantially, if at all, to its effects. This is due to their inactivity pharmacologically and/or, most often, to their extremely low concentrations.
Anhalamine
Found to be hardly active as anticonvulsant, tranquilizer or muscle relaxant by Brossi et al. 1966
Anhalidine
Found to be hardly active as anticonvulsant, tranquilizer or muscle relaxant by Brossi et al. 1966
Anhalonidine
Probably does not contribute to the pharmacology as it is one fourth as active as pellotine. Shulgin 1973
Heffter found doses of 20-25 mg of the hydrochloride produced narcosis in frogs followed by increased excitability. Complete paralysis was produced by larger dosages. A curarizing effect was caused by dosages of 30 to 50 mg. No significant effects were seen in mammals. Heffter 1898a
Said to produce slight sleepiness and a dull sensation in the head. LaBarre 1975 citing Rouhier’s Monographie pp. 227-232.
Found to be hardly active as anticonvulsant, tranquilizer or muscle relaxant by Brossi et al. 1966.
Anhalonine
Heffter 1898a found 5-10 mg injected into frogs produced an increase in the reflex excitability after a phase of paresis. Similar action was noted in rabbits but hyperexcitability was predominate. (Heffter also described other effects.)
Hordenine
Active as a stimulant [Bruhn & Bruhn 1973] but a 100 mg. dose was found by Heffter to be inactive. [Ott 1993] Hordenine may potentially contribute some activity as a norepinephrine reuptake inhibitor: Barwell et al. 1989. However, the extent of its actual contribution remains to be studied.
As Todd found this present only in the roots it may be doubtful that it contributes to the pharmacology of peyote although the claim from some users that they get mroe when eating the roots might merit evaluation. It is presently unknown whether the reported presence of hordenine in peyote buttons by other researchers reflects its occurrence in the tops during normal times of traditional harvest (perhaps before use as a biosynthetic precursor) versus Todd’s analysis occurring during June or whether it is due to the presence of roots or partial roots on the plants these other workers analyzed. (Some other workers did analyze WHOLE plants during their work.
See McLaughlin & Paul 1965, 1966 & 1967 and Rao 1970.
McLaughlin & Paul 1965 purchased their material from Penick.
McLaughlin & Paul 1965 was cited by McLaughlin & Paul 1966 for their procedure in processing the plants. In their 1966 work on biosynthesis they used plants obtained from Mexico which were maintained in a greenhouse.]
Found to cause paralysis of the CNS in frogs without previous excitation by Heffter 1894a.
Small doses have no effect on blood circulation but larger ones cause hypertension and accelerated pulse. Very large doses cause death by respiratory arrest.
Pressure effect is not of central origin but is due to stimulation of cardiac muscle. [Rietschel 1937a & 1937b]
Less active than adrenaline, more similar to ephedrine than adrenaline.
Other researchers reported a nicotine like action [Raymond-Hamet 1933a, 1933b & 1939 and Ludueña, as cited in Reti 1959]
Large doses decrease or reverse the hypertensive action of adrenaline. [Raymond-Hamet 1936]
Reported highly antiseptic and to have inhibiting effect on some soluble ferments. [Camus 1906a-d]
Comments partially adapted from Kapadia & Fayez 1970
The antibacterial and wound healing reputation of peyote and other cacti has been attributed to the presence of hordenine. See:
McCleary 1960 who studied the effects of a water soluble crystalline material extracted from peyote, which they named peyocactin, in vitro on 18 penicillin resistant strains including Staphylococcus aureus and Staphylococcus pyogenes. It inhibited all strains.
McCleary & Walkington 1964 found inhibitory effects in vivo on
mice inoculated with toxic strains of S. aureus. Found that other cacti were effective on some strains but none were as widely effective as peyote.
Rao 1970 showed that peyocactin and hordenine were identical.
Hordenine has well known antibacterial properties and was generally assumed to be the reason for the bacterial inhibition observed by McCleary above. It should be noted that in spite of peyote’s greater activity in this regard, other cacti they evaluated have been found to have higher hordenine contents. While most people have assumed that the activity is due solely to hordenine, this suggests that the matter is not yet cut and dried and some study might be worthwhile.
McLaughlin & Paul 1966 also found in vitro antibiotic activity against a broad range of microorganisms but were unable to document any significant in vivo activity.
Lophophorine
“…is highly toxic and produces strychnine-like convulsions at 12 mg./kg. doses but it produces nausea in human being at much lower doses.“ [Ott 1993 citing Anderson 1980]
Heffter 1898a “found a 20 mg. dose of lophophorine to produce vasodilation and headache.” [Ott 1993]
Shulgin 1973 & 1976 noted that all toxicity data and the assertions of its “highly toxic” nature is based on animal studies and human evaluations limited to Heffter’s single published report.
Administration of the alkaloid was said to produce an accentuated sickening feeling in the back of the head after 15 minutes, accompanied by hotness, blushing of the face and a slight slowing of the pulse. The effects are said to disappear after 40 minutes. [LaBarre 1975 citing Rouhier’s Monographie 227-232 who was referring to Heffter.]
Heffter found that 0.25-1 mg of injected hydrochloride produced a lengthy tetany in the frog. The increased excitability may last for several days but the animal recovers. (He noted no apparent action on the isolated frog heart.)
In rabbits hyperexcitability and accelerated respiration were noted at 7 mg/kg. Tetany was induced at 12.5 mg/kg and death at 15-20 mg/kg.
Intravenous injection of 2.5 mg increases blood pressure but higher doses are hypotensive, lacking a specific action on the heart. [Heffter 1898a]
Pellotine
Sedative effects at 50 mg. levels in adult humans. From Ott 1993
Temporary convulsion were caused in frogs, dogs and cats by dosages of 5-10 mg. [Ott 1993 citing Heffter 1898a]
Said to reduce the pulse by approximately a quarter in about an hour. Reported to cause heaviness of the eyelids, sensation of fatigue and an aversion to all physical and mental effort. [LaBarre 1975 citing Rouhier’s Monographie pp. 227-232]
Believed by some to be useful in man as a relatively safe narcotic. [Kapadia & Fayez 1970 referred to authors cited by Joachimoglu & Keeser 1924]
It was found to be hardly active in animals as anticonvulsant, tranquilizer or muscle relaxant by Brossi et al. 1966
Alkaloids identified in peyote
More than 70 alkaloids have been published in the literature but some of those are clear errors, others have been questioned or lack confirmation. Only around 63 of those are actually confirmed.
Candicine and O-methylpellotine are disputed, the first as other workers were unable to identify it and the second as it apparently is in L. diffusa but not L. williamsii.
One could also question 1,2-Dimethyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt as it was was identified entirely by UV and comparison with similar structures.
The following list was organized after Anderson but has been updated and expanded to include a summation of the available reports for each alkaloid.
For physical data: please see the book “The Cactus Alkaloids”
Mono-oxygenated phenethylamines:
Tyramine
tlc
McLaughlin & Paul (1966) Lloydia, 29: 315.
(0.001% dry wt: McLaughlin & Paul 1966; trace: Lundström 1971a.
Also in Habermann 1978b (from Štarha nd)
N-Methyltyramine
tlc, mp, mmp, ir
McLaughlin & Paul (1966) Lloydia, 29 (4): 315-327.
(0.012% dry wt: McLaughlin & Paul 1966; trace: Lundström 1971a.
Hordenine
tlc, mp, mmp, ir
McLaughlin & Paul (1965) Journal of Pharmaceutical Sciences, 54 (4): 661.
(Confirmed in McLaughlin & Paul (1966) Lloydia, 29 (4): 315-327.)
(0.6-0.7% dry wt: Lundström 1971b; (0.008% dry wt.) McLaughlin & Paul 1966; Todd 1969 found it only in roots (tlc).
[Also in Habermann 1978b (from Štarha nd)]
[8% of total alkaloid content: Lundström 1971b]
Candicine
(tlc) Presence in peyote is in question
McLaughlin & Paul (1966) Lloydia, 29: 315-327. (Suspected presence based on tlc.)
Kapadia et al. 1968 could not confirm. Found other quaternary alkaloids but were unable to find candicine. Nor could Davis et al. 1983
Dioxygenated phenethylamines:
Dopamine
glc, gc-ms<
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a)
Epinine
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a)
4-Hydroxy-3-methoxyphenethylamine
(3-Methoxytyramine)
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a)
N-Methyl-4-hydroxy-3-methoxyphenethylamine
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a; <0.5% of total alkaloid content: Lundström 1971b]
N,N-Dimethyl-4-hydroxy-3-methoxyphenethylamine
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a; 0.5-2% of total alkaloid content: Lundström
1971b)
3,4-Dimethoxyphenethylamine
glc, gc-ms
Lundström & Agurell (1968) Journal of Chromatography 36 (1): 105-108.
(trace: Lundström & Agurell 1968 and Lundström 1971a. Also in Habermann 1978b: from Štarha nd)
N-Methyl-3,4-dimethoxyphenethylamine
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a)
3,4-Methylenedioxyphenethylamine
(Homopiperonylamine)
HPLC
Bruhn et al (2008)
(Reportedly observed but lacking isolation & characterization and independent confirmation.)
N,N-Dimethyl-3,4-methylenedioxyphenethylamine
(Lobivine)
HPLC
Bruhn et al (2008)
(Reportedly observed but lacking isolation, characterization and independent confirmation.)
Trioxygenated phenethylamines and related amides:
3,4-Dihydroxy-5-methoxyphenethylamine
glc, gc-ms
Lundström (1971)a Acta Chemica Scandinavica, 25 (9): 3489-3499
(trace: Lundström 1971a)
3-Hydroxy-4,5-dimethoxyphenethylamine
(3-Demethylmescaline)
gc, gc-ms
Kapadia et al. (1969)a Journal of Pharmaceutical Sciences, 58 (9): 1157-1159.
Agurell & Lundström (1968) The Chemical Society, London. Chemical Communications, 24: 1638-1639.
(5% of total alkaloid: Agurell & Lundström 1968; 1-5% of total alkaloid content in fresh material: Lundström & Agurell 1971b. Also (identified) by Kapadia et al. 1969a and Agurell & Lundström 1968)
N-Methyl-3-hydroxy-4,5-dimethoxyphenethylamine
gc, gc-ms
Lundström (1971)c Acta Pharmaceutica Suecica, 8 (5): 485-496
(trace: Lundström 1971c)
N,N-Dimethyl-3-hydroxy-4,5-dimethoxyphenethylamine
gc, gc-ms
Lundström (1971)c Acta Pharmaceutica Suecica, 8 (5): 485-496
(0.04% dry weight i.e. 0.5% of 8% total alkaloid content: Lundström 1971c; 0.5% of total alkaloid content: Lundström 1971b)
N-Formyl-3-hydroxy-4,5-dimethoxyphenethylamine
(N-Formyl-3-demethylmescaline)
gc, gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
N-Acetyl-3-hydroxy-4,5-dimethoxyphenethylamine
(N-Acetyl-3-demethylmescaline)
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
3-Methoxy-4,5-methylenedioxyphenethylamine
(Lophophine)
HPLC
Bruhn et al (2008)Lacking isolation & characterization. In need of confirmation.
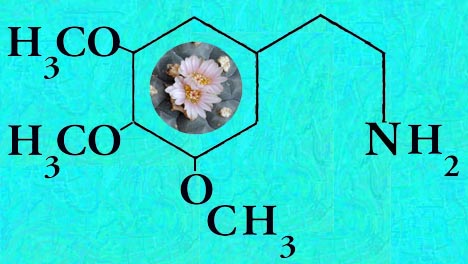
Mescaline (3,4,5-Trimethoxyphenethylamine)
mp, mmp
Heffter (1896)a Berichte der Deutschen Chemischen Gesellschaft, 29: 216-227 (original isolation) but the structure was not actually determined until Späth (1919) Monatshefte fuer Chemie, 40: 129-154.
([0.10-]0.9-6.0[-6.3]% dry wt. has been reported [Note 22] [Anonymous 1959, Heffter 1896a, Lundström 1971b, Martin & Alexander 1968 & Siniscalco 1983);
Anderson 1980 cited Kelsey 1959 (0.9%), Bergman 1971 (1.5%), Fischer 1958 (3%), Heffter 1896a (4.6-5.6%[-6.3%])];
2.4-2.7 % dry (~400 mg. per 16 grams of dried cactus) Ott 1993 citing Bruhn & Holmstedt 1974 and Lundström 1971b
[Crosby & McLaughlin 1973 stated peyote can reach 6% but rarely exceeds 1% (dry wt.)]
[Tops>>Roots; Todd 1969 [Note 23]]
Siniscalco 1983 reported the isolation of 0.10% (well irrigated),
0.93% (grafted) and up to 2.74% dry weight (after 6 months of dry conditions) from plants cultivated in Italy; 0.1 to 0.2% by fresh weight is common
Friends with extraction experience found fresh Texas plants to average 0.2% during 1970s
75-125 mg of HCl was recovered from 70-140 gm plants greenhouse grown in northern Europe. Lundström & Agurell 1971b (This approaches 0.1% by fresh weight) [Also in Habermann 1978a & 1978b (from Štarha nd)] [30% of total alkaloid content: Lundström 1971b]
[As L. williamsii var. typica Croizat: 0.709% (± 0.032) dry wt.
Habermann 1978a (from Štarha 1997)]
N-Methylmescaline
mp, mmp
Späth & Bruck (1937) Berichte der Deutschen Chemischen Gesellschaft, 70 (12): 2446-2450.
(0.24% dry wt., 3% of total alkaloid: Lundström 1971b)
N-Formylmescaline
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
N-Acetylmescaline
mp, mmp
Späth & Bruck (1938) Berichte der Deutschen Chemischen Gesellschaft, 71 (6): 1275-1276.
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Späth & Bruck 1938 and Kapadia & Fales 1968a)
Tetrahydroisoquinolines and related amides:
Anhalamine
mp, mmp
Kauder (1899) Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft, 237: 190-198.
(0.1-0.7% dry wt. has been reported: Späth & Becke 1935b and Lundström 1971b; Also in Habermann 1974a (from Štarha nd); 8% of total alkaloid content: Lundström 1971b)
N-Formylanhalamine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
N-Acetylanhalamine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Isoanhalamine
gc, gc-ms
Lundström (1972) Acta Chemica Scandinavica, 26: 1295-1297.
(trace: Lundström 1972)
Anhalidine
mp, mmp
Späth & Beck (1935)b Berichte der Deutschen Chemischen Gesellschaft, 68 (5): 944-945.
(0.001% dry wt: Späth & Becke 1935b; 0.16% dry wt. i.e. 2% of 8% total alkaloid content: Lundström 1971b)
Anhalotine (4° amine isolated as Iodide)
ir, nmr, uv
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
(0.0003% dry wt: Kapadia et al. 1968)
Isoanhalidine
gc, gc-ms
Lundström (1972) Acta Chemica Scandinavica, 26: 1295-1297.
(trace: Lundström 1972 & 1971b)
Anhalinine
mp, mmp
Späth & Beck (1935) Berichte der Deutschen Chemischen Gesellschaft, 68 (3): 501-505.
(0.01% dry wt: Späth & Becke 1935b; 0.04% dry wt., 0.5% of total alkaloid content: Lundström 1971b)
N-Formylanhalinine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968)
Anhalonidine
mp, mmp
Heffter (1896)a Berichte der Deutschen Chemischen Gesellschaft, 29: 216-227.
(1.12% dry wt., 14% of total alkaloid content: Lundström 1971b; Also
in Habermann 1974a: from Štarha nd)
Pellotine
mp, mmp
Heffter (1894)b Berichte der Deutschen Chemischen Gesellschaft, 27: 2975-2979.
Kauder, E. (1899) Archiv der Pharmazie und Berichte der Deutschen Pharmazeutischen Gesellschaft, 237: 190-198.
(±)-Pellotine
UV, IR, NMR
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
(-) Pellotine
UV, CD
Cymerman Craig, J. et al. (1977) Journal of the American Chemical Society 99 (24): 7996-8002.
1.36% dry weight: Lundström 1971b;
Also (%?) Habermann 1974a, 1978a & 1978b: from Štarha nd;
17% of total alkaloid content: Lundström 1971;
As L. williamsii var. typica: 0.296% (± 0.065) Habermann 1978a: from Štarha in Grym 1997.
Peyotine (4° amine isolated as Iodide)
Pellotine methiodide
mp, UV, IR
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
(0.00015% dry wt: Kapadia et al. 1968)
N-Formylanhalonidine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Isoanhalonidine
gc, gc-ms
Lundström (1972) Acta Chemica Scandinavica, 26: 1295-1297.
(trace: Lundström 1972)
Isopellotine
gc, gc-ms
Lundström (1972) Acta Chemica Scandinavica, 26: 1295-1297.
(0.04% dry weight, 0.5% of total alkaloid content: Lundström 1971b)
S-(+)-O-Methylanhalonidine
O-Methyl-d-anhalonidine
mp, mmp
Späth & Bruck (1939) Berichte der Deutschen Chemischen Gesellschaft, 72 (2): 334-338.
(0.04% dry wt., <0.5% of total alkaloid content: Lundström 1971b)
N-Formyl-O-methylanhalonidine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
O-Methylpellotine
gc, gc-ms (using L. diffusa)
[Bruhn & Agurell (1975) Phytochemistry,14: 1442-1443.]
Presence in L. williamsii is in doubt. It is included by Mata & McLaughlin 1982 but they do not list individual references for the compounds.
Bruhn & Agurell believed that it is unique to L. diffusa but it was later it was found in Pachycereus weberi.
I am still reviewing Mata & McLaughlin’s references in case someone found this as a trace component in peyote but that does not presently appear to be likely. Štarha did not detect it in L. fricii or L. jourdaniana but DID report it in L. koehresii. Obviously Štarha’s work was not available to Mata & McLaughlin in 1982
6,7-Dimethoxy-8-hydroxy-3,4-dihydroisoquinoline
mp, UV, IR, NMR, MS
Fujita et al. (1972) Yakugaku Zasshi, 92 (4): 482-489.
(Journal of the Pharmaceutical Society of Japan)
(0.0008% fresh weight: Fujita et al. 1972; as L. williamsii var. caespitosa)
2-Methyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt
mp, uv, IR, NMR, MS
Fujita et al. (1972) , 92 (4): 482-489.
(Journal of the Pharmaceutical Society of Japan)
(0.001% fresh weight: Fujita et al. 1972: as L. williamsii var. caespitosa)
1-Methyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinoline
mp, UV, NMR, ms
Fujita et al. (1972) , 92 (4): 482-489
(0.0001% fresh weight: Fujita et al. 1972: as L. williamsii var.
caespitosa)
1,2-Dimethyl-6,7-dimethoxy-8-hydroxy-3,4-dihydroisoquinolinium inner salt
UV
Fujita et al. (1972) , 92 (4): 482-489.
(0.00008% fresh wt: Fujita et al. 1972: as L. williamsii var. caespitosa)
Lophotine (4° amine isolated as Iodide)
ir, nmr, uv
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
(0.0002% dry weight: Kapadia et al. 1968)
S-(-)-Anhalonine
mp, mmp
Heffter (1896)a Berichte der Deutschen Chemischen Gesellschaft, 29: 216-227.
UV, IR, NMR
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262
(0.24% dry wt., 3% of total alkaloid content: Lundström 1971b)
S-(-)-Lophophorine
mp, mmp
Heffter (1896)a Berichte der Deutschen Chemischen Gesellschaft, 29: 216-227.
UV, IR, NMR
Kapadia et al. (1968) Journal of Pharmaceutical Sciences, 57 (2): 254-262.
(0.4% dry wt: Lundström 1971b;
0.5% dry wt: Heffter 1898c.
[Also in Habermann 1974a (from Štarha nd)]
5% of total alkaloid content: Lundström 1971b;
Appeared to be the major alkaloid in 2 sorts of summer collected plants: tlc by Todd 1969)
N-Formylanhalonine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
N-Acetylanhalonine
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Peyophorine
tlc, gc, ir, ms, mp
Kapadia & Fales (1968)b Journal of Pharmaceutical Sciences,. 57 (11): 2017-2018.
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a; 0.04% dry wt., 0.5% of total alkaloid content: Lundström 1971b)
Conjugates with Krebs Acids:
Peyoxylic acid
gc
Kapadia & Fayez (1973) cites Kapadia et al. (1969) 116th Meeting of the American Pharmaceutical Association, Montreal, Canada. May 18-22. “Identification and synthesis of 3-demethylmescaline, a plausible intermediate in the biosynthesis of the cactus alkaloids.”
Kapadia & Fayez (1970) cited “Kapadia, Rao, Leete, Fayez, Vaishnav and Fales, to be published.” i.e. Kapadia et al. (1970)b Journal of the American Chemical Society 92 (23): 6943-6951.
(trace: Kapadia et al. 1970)
O-Methylpeyoxylic acid
mp, NMR
Kapadia et al. (1973) Journal of Heterocyclic Chemistry, 10 (1): 135-136.
(trace: Kapadia et al. 1973)
Peyoruvic acid
gc
Kapadia et al. (1970)b Journal of the American Chemical Society, 92 (23): 6943-6951.
(trace: Kapadia et al. 1970)
O-Methylpeyoruvic acid
mp, NMR
Kapadia et al. (1973) Journal of Heterocyclic Chemistry, 10 (1): 135-136.
(trace: Kapadia et al. 1973
Mescaloxylic acid
tlc, gc-ms, synthesis, NMR, MS
Kapadia & Hussain (1972) Journal of Pharmaceutical Sciences, 61 (7): 1172-1173.
Kapadia et al. (1971) 118th Meeting of the American Pharmaceutical Association, San Francisco, California, March 27-April 2. “Some newer synthetic cactus alkaloid analogs.”
(trace: Kapadia & Hussain 1972)
Mescaloruvic acid
tlc, gc-ms, synthesis, NMR, MS
Kapadia & Hussain (1972) Journal of Pharmaceutical Sciences, 61 (7): 1172-1173.
Kapadia et al. (1971) 118th Meeting of the American Pharmaceutical Association, San Francisco, California, March 27-April 2. “Some newer synthetic cactus alkaloid analogs.”
(trace: Kapadia & Hussain 1972)
Mescaline succinamide
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Highet 1968)
Mescaline malimide
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Mescaline maleimide
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Mescaline citrimide
gc-ms, and ir, nmr and ms of synthetic
Kapadia et al. (1970)a Lloydia, 33 (4): 492.
Kapadia & Fayez (1970) cite Kapadia et al. “11th Ann. Meet. Amer. Soc. Pharmacognosy (Vienna, Austria) July 1970, To be published.” i.e. Kapadia et al. (1970)a Lloydia, 33 (4): 492.
(trace: Kapadia et al. 1970)
Mescaline isocitrimide lactone
gc-ms, and ir, nmr and ms of synthetic
Kapadia et al. (1970)a Lloydia, 33 (4): 492.
Kapadia & Fayez (1970) cite Kapadia et al. “11th Ann. Meet. Amer. Soc. Pharmacognosy (Vienna, Austria) July 1970, To be published.” i.e. Kapadia et al. (1970)a Lloydia, 33 (4): 492.
(trace: Kapadia et al. 1970)
Peyoglutam
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Mescalotam
gc-ms
Kapadia & Fales (1968)a The Chemical Society, London. Chemical Communications, 24: 1688-1689.
(trace: Kapadia & Fales 1968a)
Pyrrole derivatives:
Peyonine
gc, ms, ir, nmr, tlc, glc, uv, mmp, synthesis
Kapadia & Shah (1967) Lloydia, 30: 287. (Proceedings.)
Kapadia & Highet (1967) Lloydia, 30: 287-288 (Proceedings.)
Kapadia & Highet (1968) Journal of Pharmaceutical Sciences, 57: 191-192.
(trace: Kapadia & Highet 1968
Peyoglunal
gc-ms, ir, nmr, ms, color reactions, synthesis
Kapadia et al. (1970)a Lloydia, 33 (4): 492.
(trace: Kapadia et al. 1970)
Other alkaloids:
Choline
tlc, gc, ir
Strongly alkaline viscous liquid. 123 mg from 2.3 kg dried peyote.
Identified by mp and mmp of picrate and IR,
Kapadia et al. (1968) Journal of Pharmaceutical Sciences,. 57 (2): 254-262.
(0.005% dry wt: Kapadia et al. 1968
See Anderson 1980 pages 191-203 and Menachery et al. (1986) (THIQ); both have line drawings of structures. (See also Cactus Chemistry By Species)
Two other inclusions appear in some listings of peyote alkaloids:
N-(3,4,5-Trimethoxyphenethylamine)-alanine /h2>
[Synonym for Mescaloruvic acid; See Kapadia & Hussain 1972a]
N-(3,4,5-Trimethoxyphenethylamine)-glycine
[Synonym forMescaloxylic acid; See Kapadia & Hussain 1972a]
Do not confuse either compound with the
3,4,5-Trimethoxyphenethyl-glycine which Sethi et al. 1973 synthesized
for use as a reference standard but were unable to observe in the
plant.
[Note: 3,4,5-Trimethoxyphenylalanine
3,4,5-Trimethoxyphenethylglycine.]
Other compounds reported from Peyote
Serotonin was claimed in hplc by Gennaro et al. 1996. This identity was never conclusively proven and it has not been confirmed.
Glucaric acid (saccharic acid) (tlc by Kringstad & Nordal 1975).
Calcium oxalate (the forms and degree of hydration have not been established)
Users of fresh peyote have observed it as well due to it being readily perceived as sand or grit present inside of the flesh. Oxalate is sometimes present in appreciable quantities.
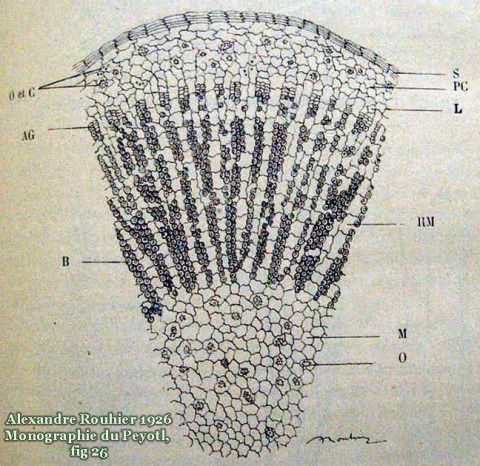
Rouhier 1926 observed the presence of oxalate crystals in his histological study of the plant. These are labelled “O” in the drawing below; “C” is said to indicate the shards created by the action of the microtome when making the thin section slice.
Oxalate appears to be present in the form of druses (whewellite?), crystal sand and as additional forms. Rouhier commented on “oursins d’oxalate de chaux [weddellite?] et vaisseaux spiralés” being present in the flesh in addition to “macies d’oxalate de calcium“.
Spiky crystals inside of cacti are often Weddellite (CaC2O4•2H2O) and the rounded druses Whewellite (CaC2O4•H2O) but the nature of the biominerals that exist inside of peyote flesh apparently remains unstudied. (Weddelite is extremely rare in nature outside of cacti biominerals and as a component of kidney stones but it is common in both of those.)
Biosynthetic studies
Studies and route proposals for mescaline and peyote alkaloids(s):
Agurell & Lundström 1968
Agurell et al. 1967
Basmadjian & Paul 1971
Battersby et al. 1967
Kapadia & Fayez 1970
Khanna et al. 1969
Leete 1959 & 1966
Lundström 1971a & 1971b
Lundström & Agurell 1968b, 1969, 1971 & 1972
McLaughlin & Paul 1967
Paul 1973
Paul et al. 1969a & 1969b
Reti 1950
Rosenberg & Stohs 1974 [Comparative utilization studies for tyrosine in protein and alkaloids biosynthetic pathways. They determined the utilization of tyrosine for incorporation into alkaloids is three times the rate of incorporation into protein.]
Rosenberg et al. 1967 & 1969
Peyote alkaloids other than mescaline:
Battersby et al. 1968
Kapadia et al. 1970b
Khanna et al. 1970 [Radiolabeled precursor incorporation studies.]
Leete & Braunstein 1969
Lundström 1971c & 1972 [the latter is not a biosynthetic study per se but does offer some supportive evidence]
McFarlane & Slaytor 1972a [A point on biosynthesis of anhalonidine] & 1972b [Biosynthesis of 3,4-Dimethoxyphenethylamine]
For a review of tetrahydroisoquinolines in peyote and other cacti see pp. 256-276 in:
Jan Lundström (1983) “Simple Isoquinoline Alkaloids.” pp. 255-327 (Chapter 6) in: Arnold Brossi (Ed.) The Alkaloids. Chemistry and Pharmacology. Volume 21.
See also:
Mary D. Menachery et al. (1986) Journal of Natural Products, 49 (5): 745-778. “Simple Isoquinolines” (for a review of physical data and distribution.)