My blood might not be the most interesting topic but among the local residents of the forest are several microbes that are capable of causing human problems. They are all largely carried by ticks although fleas can also transmit Bartonella.
Tick imagery – caution about this link: some of the tick images are graphic and might be objectionable to some viewers.
I am shutting down another website and decided to move some of the material from there to here due to believing it had some value for education about Babesia and ticks.
Updates follow the original (edited) account below.
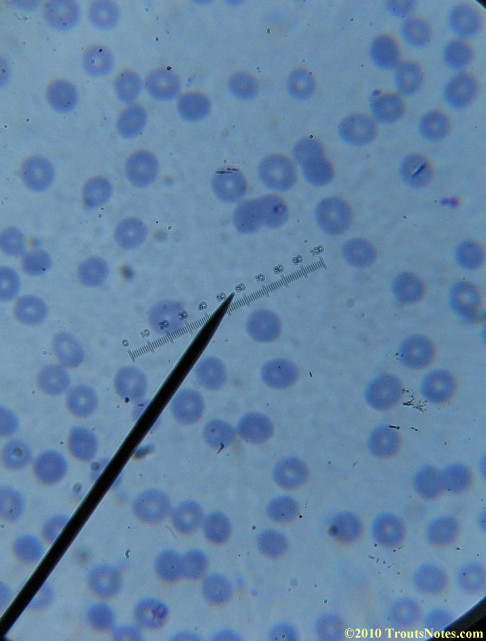
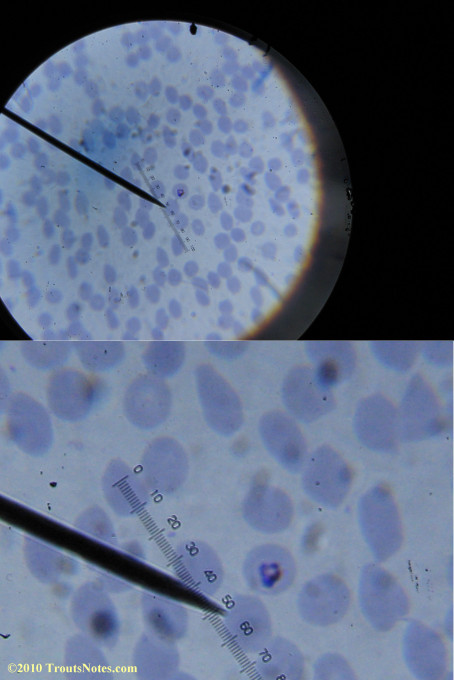
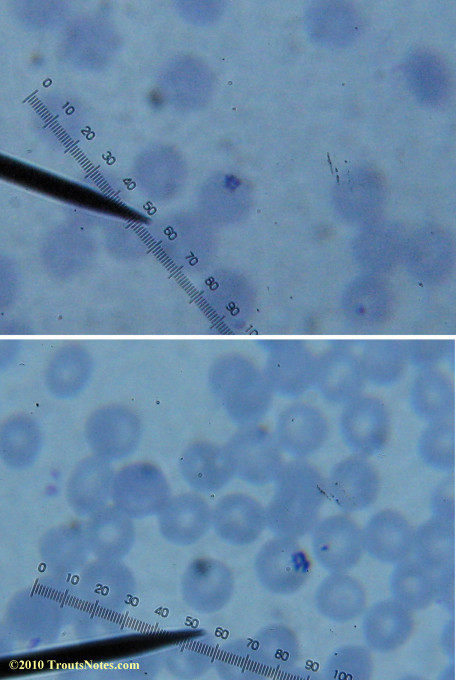
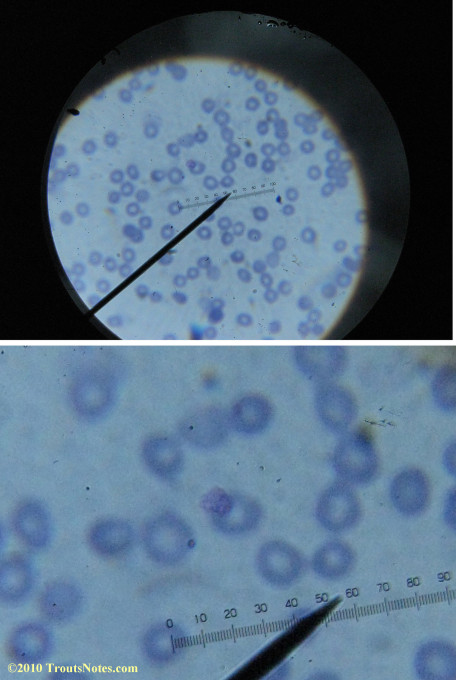
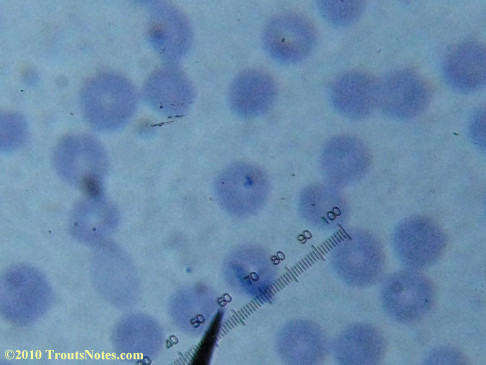
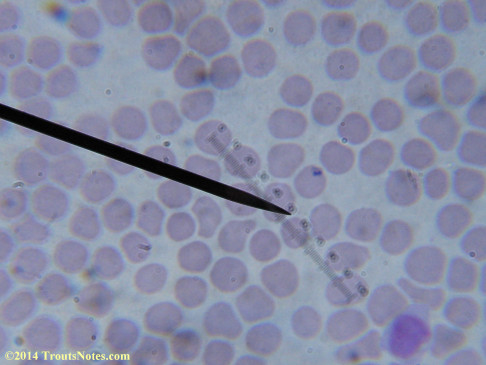
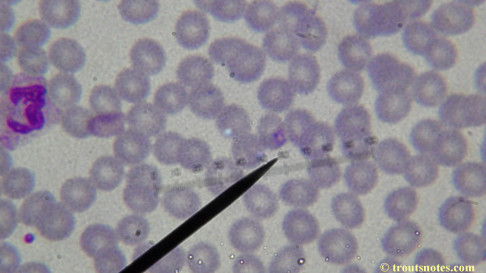
My red blood cells in early October 2011
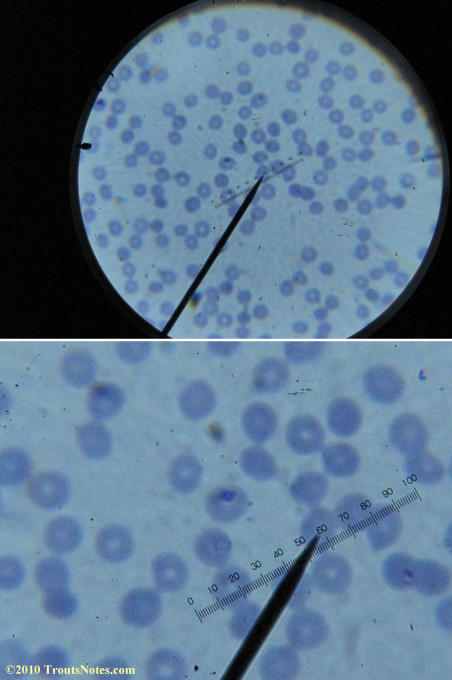
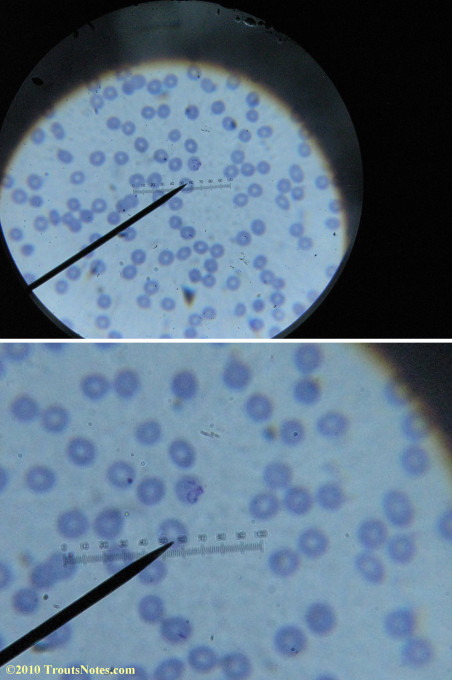
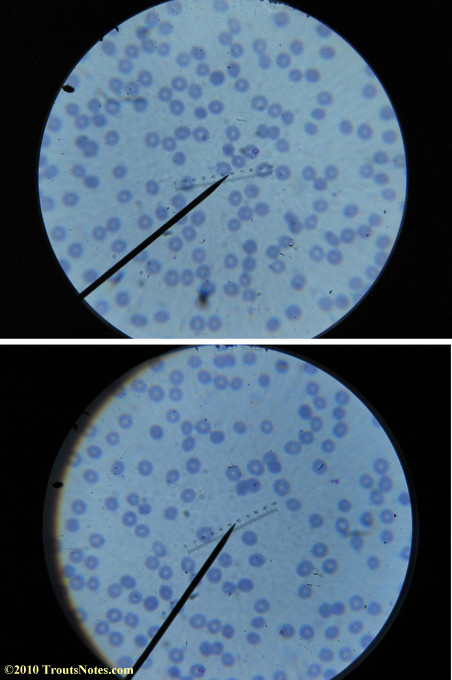
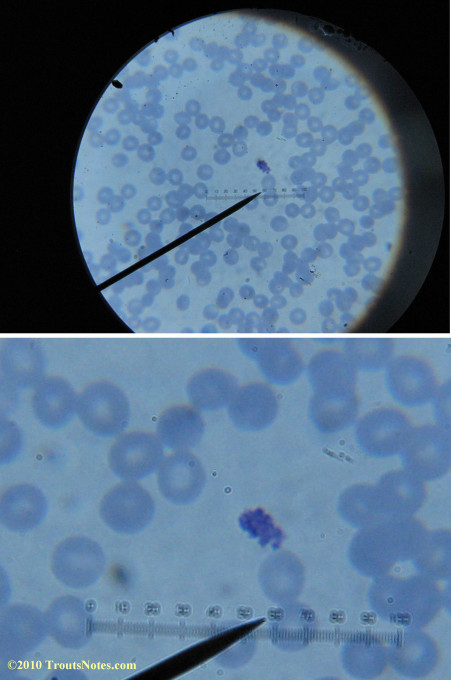
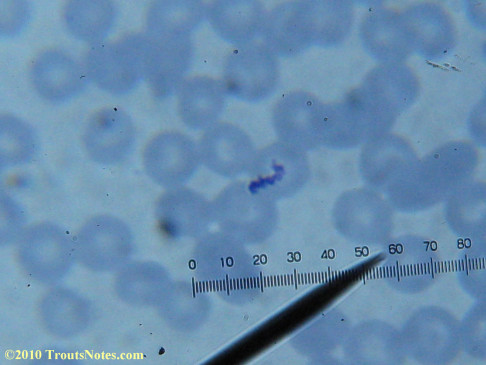
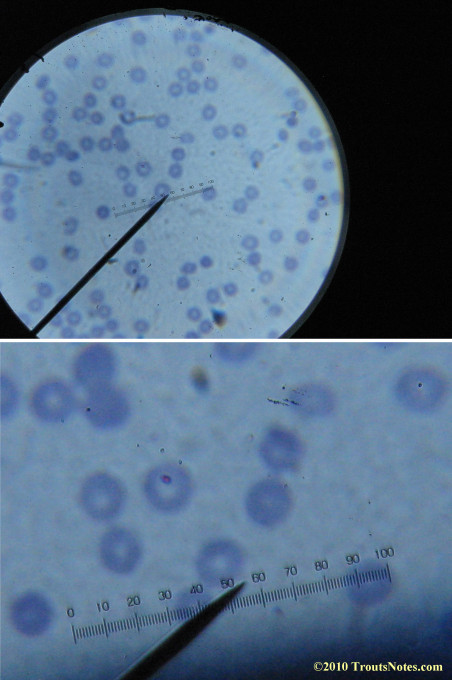
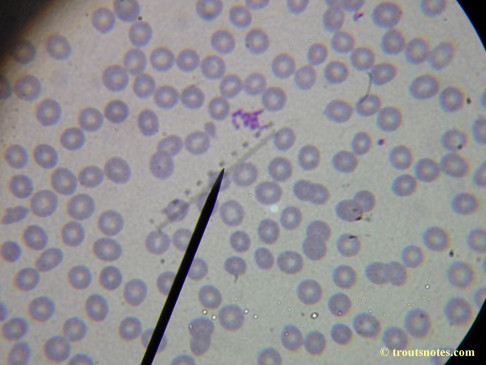
What sure looks like Babesia to my amateur eyes (the purple rings in the red blood cells) — and some images of who knows what can be seen below. (Images were accidently mis-dated 2010 during labeling. My adventures with lyme testing actually began in late 2010 but the images that are presently labeled 2010 are all from 2011.)
I think that some of these photographs nicely support the preexisting notion (shared by both my current and previous doctor) that I have Babesia duncani.
My results in 2011 came back with negative results in all IGeneX testing performed for Babesia microti and Babesia duncani. Including B. microti Antibody G/M, B. duncani IFA (G/M), B. microti/duncani PCR, and their Babesia FISH (RNA) test. My previous doctor (a Lyme literate MD) commented that in his experience symptomology was of greater usefulness for diagnosing Babesia than blood tests and the results below do add some weight to that opinion.
Please compare my blood imagery to the images of Babesia at:
http://www.dpd.cdc.gov/dpdx/html/imagelibrary/Babesiosis_il.htm
Particularly the one posted there for Babesia duncani.
This is the species prevalent in my local area of Mendocino County, California.Another valuable source of images for comparison is this PDF:
http://www.cdc.gov/dpdx/resources/pdf/benchAids/Babesia_benchaid.pdfThere are also many images of Babesia in human blood in the PDF at
http://www.personalconsult.com/free/BabesiaLabGuide.pdfAdditional comments follow the images below.
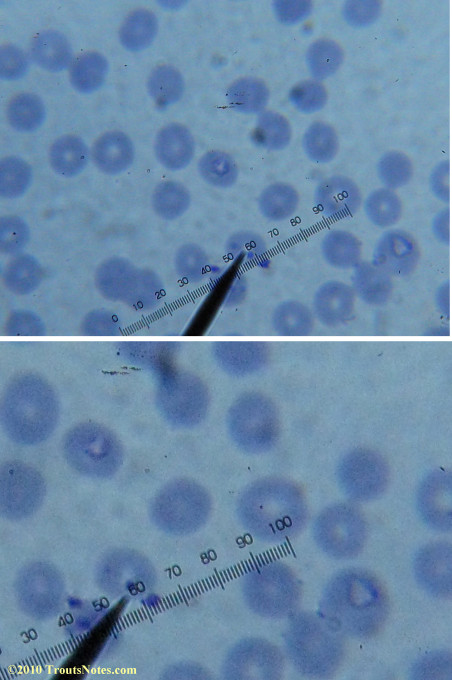
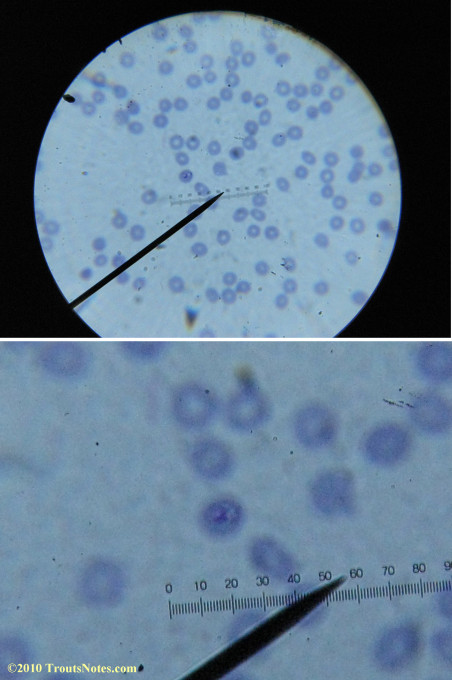
Most of the images came from one slide and all of the 2010 images came from within three slides.
I was really lucky to be so productive on the first slide as it would take a lot of focused work to thoroughly go through an entire slide of red blood cells.Images were taken by me. Lots of dust and noise is on the slides.
Visualization was with Wright’s stain and observation used a Celestron 4335 microscope at 1000X.
Images were shot using a Canon Powershot SD1100IS pointed into the eyepiece of the microscope.
Closeups were additionally 2.6 or 3.2X optical zoom from the point-and-shoot.Scale bar increments should be multiplied by 0.0004982561mm = 0.498256μm at 1000X for this microscope
(calibrated with a micrometer.)Interestingly, after posting these online, I read on the ILADS website that what would seem to have been observed should not be expected to be there.There is a comment by Dr. Burrascano concerning Babesia that “Only in early infections (less than two weeks duration) can the standard blood smear be helpful.”
See page 5 of http://www.ilads.org/files/burrascano_0905.pdfI wrote ILADS to get clarification and was directed to make inquiry of the lab director of a lyme testing lab that performs similar tests. He agreed that it was true, explained why and then told me that my slides looked like “text book slides” of Babesia. I don’t think so and think he was just a nice person being generous. I am happy enough with them though. A better microscope with camera would have been great. That would have made some nice text book slides. Still, I wrote back and thanked him for the compliment. I am curious at what I hear next, if anything. I would not be surprised to learn he thinks I am some nutcase. I can’t blame him.
The lab director of Igenex was not so generous and threatened to take action if I was discovered to be “bad-mouthing” them. Despite my embrassingly rude response to that threat (I believe my exact words were “Well f*** you lady” before hanging up on her) I would never take such action as I assume they are genuinely interested in helping people but am puzzled at their lack of interest in my results (and the lack of response from the CDC when I contacted them.)
It seems hardly any surprise that Babesia is rampant in my local area. I know easily a handful of people with positive blood tests. I am hoping that testing labs looking for Babesia will be interested in something low tech that an amateur (me) seems to have had success attempting? Especially if the literature suggests it not worth trying? Maybe I got lucky. Since it seems to be a normal, if not primary, diagnostic tool in other people’s recommendation I am perplexed and don’t know what to think. Maybe Burrascano just means it becomes dificult or more difficult after two weeks. Diagnostic manuals suggest that 200 or even 300 fields and multiple slides be examined with half an hour spent per slide when looking for Babesia. It also seems to be the usual way that diagnosticians look for Babesia so it was strange to encounter.
Thankfully, I did not know that what I was doing should not work.
I’m currently wondering if the problem is that people inspecting blood smears may need to spend more time per smear or if perhaps I caught my blood in a peak period of illness.
I would love to know why this seems to have worked for me, as reproducibility would make this very much worth knowing about. Its true I might not represent the average example.
I was under no pressure, I was not being paid anything (it actually cost me to do this) and I had some time to kill in betwen other things. Blood smears are not a part of my normal interests.I no doubt did have a bit more motivation than most people looking for Babesia in a blood smear or three?I am not a doctor and am not a diagnostician and I am an amateur lacking any experience with this. I also lack any credentials one might prefer of a person presenting blood smears as if they had some meaning so I’d suggest this is best viewed as a request for evaluation not a conclusion.However I do know how to use a microscope, have prior experience as an LRA in a biochemical research lab, and I took the time to educate myself prior to beginning. I would love to someday hear that someone else evaluated the reality of my claims.
Or maybe definitions of unusual are in order? Perhaps I am what is unusual in terms of deciding to learn how to perform my own diagnostic blood work? I certainly lack any further interest in this subject beyond recovering from it.
No positive ID as to the species in my blood has been made by anyone competent but B. duncani is probable as I live on a heavily forested property where two other residents have been diagnosed with Babesia duncanii (including positive Igenex test results for B. duncanii). Perhaps Burrascano’s comments are true for B. microti?
I don’t know but it seems worth asking the question.
If there are diagnostic labs out there that want to attempt replication:
I should empathize that despite having taken Microbiology at UT Austin quite some time ago (and performing well academically) I lacked any experience in making blood smears so began by practicing making blood smears.
I already owned a microscope so purchased a couple of Coplin jars and a small bottle of Wright’s stain.
The biggest challenge was repeatedly pricking myself with a sewing needle to provide a drop of blood – this was an act that grew more difficult with every botched smear attempt. An accidental cut on a finger tip proved far more effective due to the volume of blood it generated.
The sample was fresh blood freely flowing from the accidental laceration on one of my fingers. It was freshly applied to a slide using the outside of a capillary tube as a dripper and the testing performed as soon as it had dried.
Another slide was used to spread it as a thin blood smear as had been encountered described in the blood cytology pages at www.ruf.rice.edu.
Garcia’s description of using Wright’s stain was chosen (i.e. L.S. Garcia 2001 Diagnostic Medical Parasitology)
The smears (multiple slides were made) were allowed to air dry and were then immersed in Coplin jar containing commercial premixed Wright’s stain (from Fisher) and allowed to stand for around 20 seconds. Garcia’s instructions said 15-30 seconds but doing as many as there were slots in the jar a couple of times meant some variance in times for each. (The Wright’s stain had partially evaporated to around 75% of its original volume so was somewhat more concentrated.)
The slides were then rinsed in distilled water in another Coplin jar – additionally using a stream from a distilled water wash bottle as the number of slides increased and fouled the water in the Coplin jar .
Completed slides were allowed to dry in a vertical position inside of a loosely covered slide box overnight. Two capillary tubes were laid in the bottom of the slide box to prevent the slides from touching the cork lining during drying.
Observation under 1000X oil immersion was direct using no cover slip.
Personal observations and comments:
I had my best successes on the first slide letting intuition guide where I looked. Not real helpful perhaps for a diagnostic lab?
I was more methodical on the second and third slide and easily spent twenty minutes each to locate only one nice ring formation on each one.
Additional odd bits:
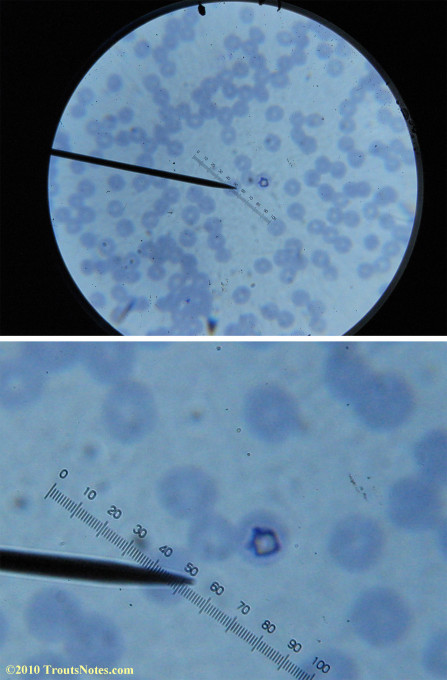
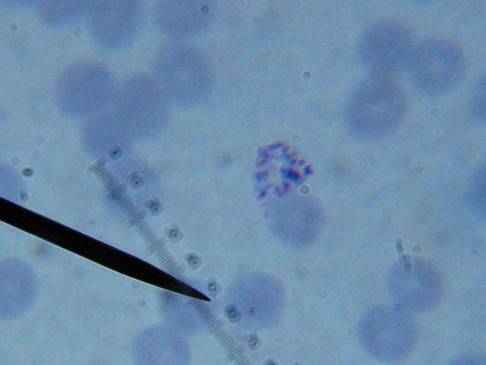
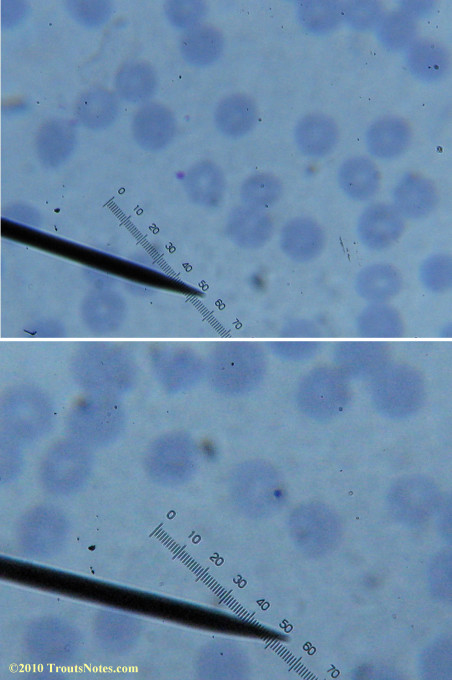
I lack training in diagnostics but found some other images interesting for sake of the odd dots and such.
More recent comments:
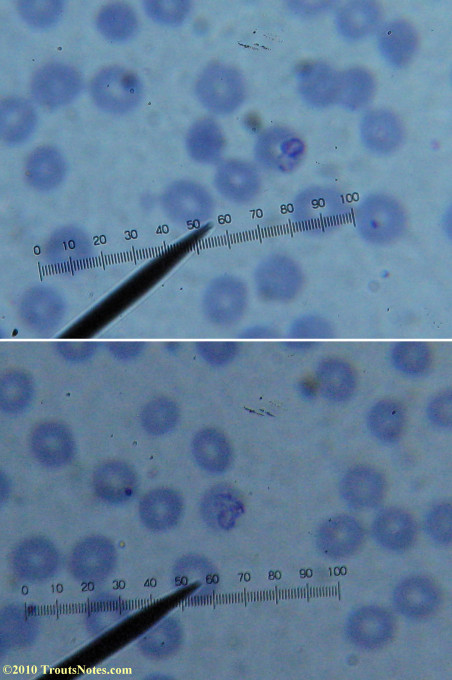
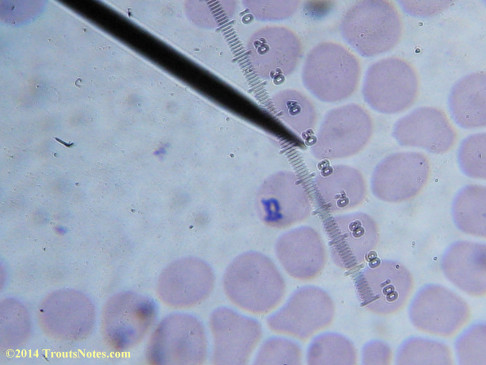
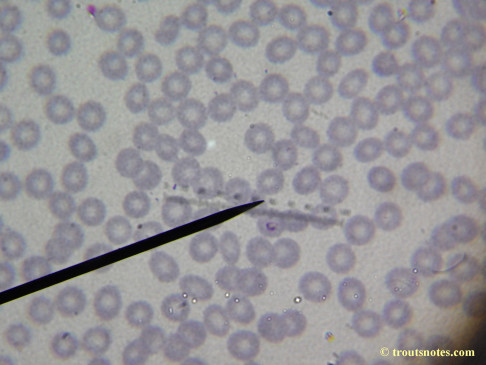
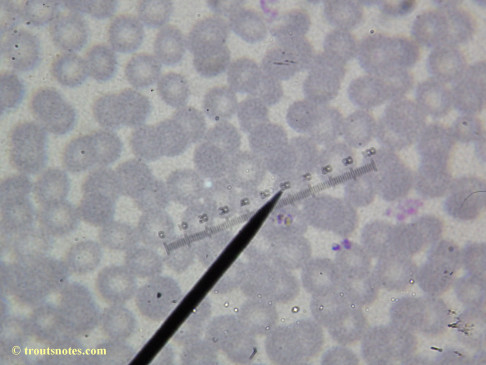
This was my blood in April 2014. I should probably look at it more often but, despite loving microscopy, I really do not like looking at my blood. It is rather tedious and when I find things that look out of place it is really disturbing to me. There have been a host of things I don’t recognize that I am not bothering to post. Frankly, blood work is something that a person should be able to pay professionals to perform. I can produce results but I do not have the training to know what I’m looking at. Despite that I do have many years of experience with using a microscope. LOTS of weird stuff can show up on slides that mean nothing at all.
There were some weird looking dots
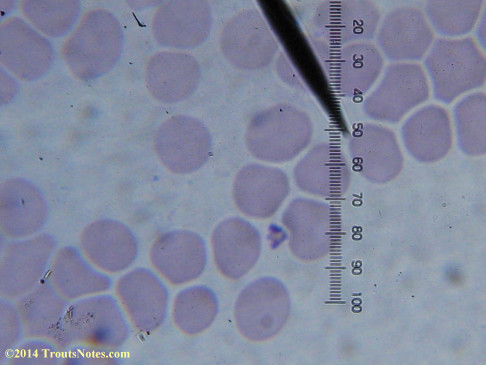
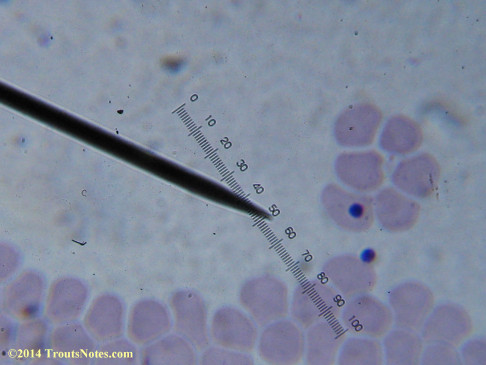
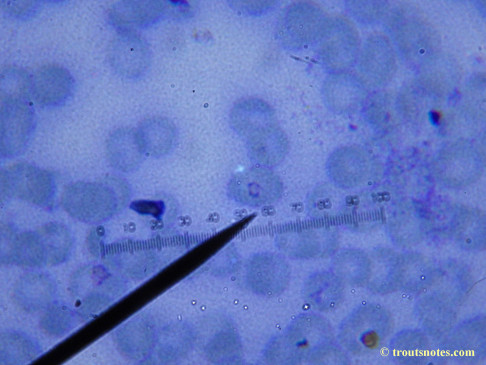
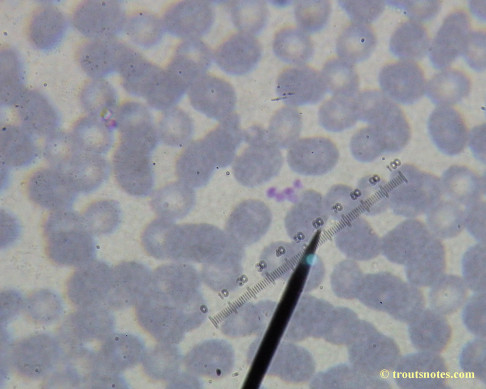
This was my blood in late May 2015.
Only a few months ago, shortly before my doctor was changed, a blood smear was supposedly read by a different diagnostic lab looking for things such as these. They reported observing nothing.
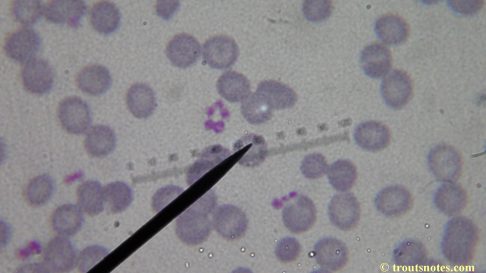
In the latest twist of my surreal now 9+ year adventure with lyme and coinfections (my first round of IGeneX tests occurred in 2010 and my inroduction to ‘lyme’ in 2006), I was recently forced to change my doctors by the new Obama Care system so have discovered myself to be starting over in seeking treatment, if not starting over in obtaining an actual diagnosis. My new doctor’s nurse practitioner told me that she had never heard of Babesia so I am not optimistic resolution will be as fast as I might prefer. My new doctor lacked interest in seeing the results of my blood work; I suspect he likely thought me strange for performing it. I decided to take another look at my blood just for a baseline in starting out just so that *I* have something to compare with that future blood work.
I’m not sure what to think. Maybe they need FRESH blood? Maybe blood should be taken when symptoms are peaking? For whatever reason I seem to be seeing unidentified rings and dots that should not be there.
I do not know what I’m seeing in the following images from 24 May 2015 but they are pretty trippy looking.